Lab Demo Iodine Clock Reaction (Chemical Change) YouTube

Recreating the Iodine Clock Reaction at Home with Vitamin C YouTube
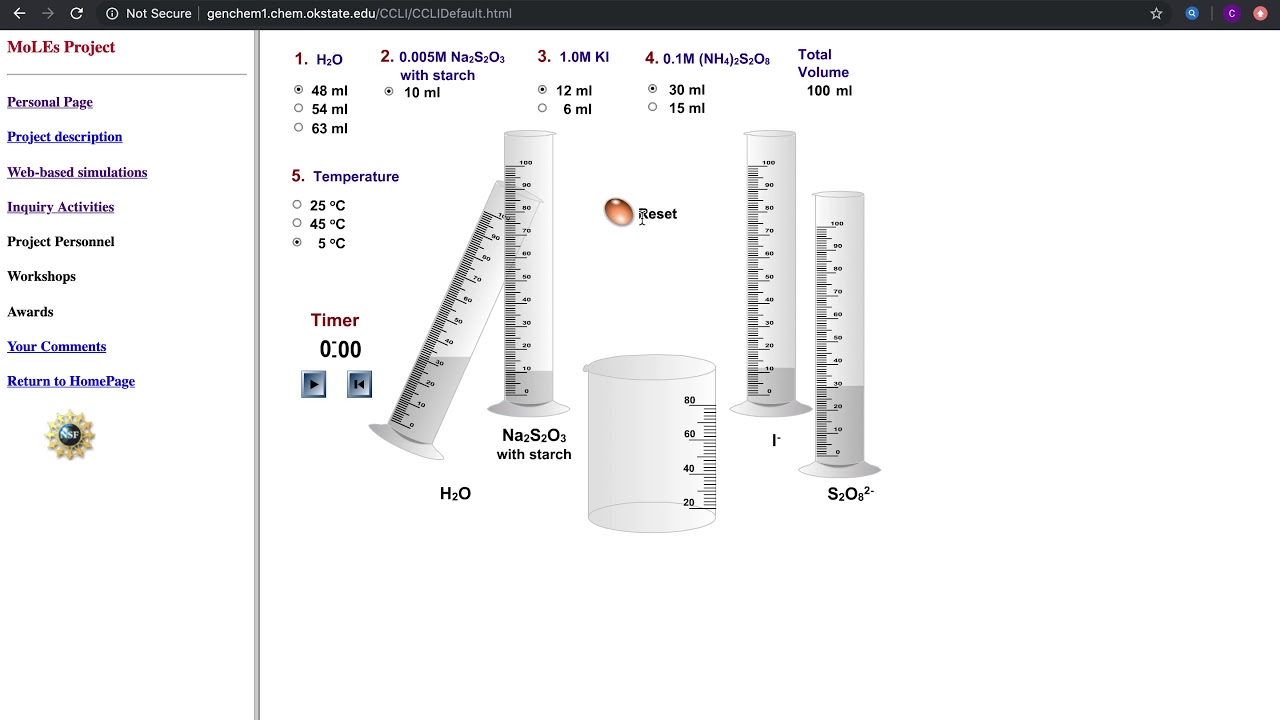
This iodine is immediately consumed by the thiosulfate ions (S 2 O 3 2-) in a pathway described by reaction (6). As soon as all of the S2O3 2- ions are consumed, the excess iodine produced in (5) is free to react with starch, turning the solution blue (7). The amount of thiosulfate ions added tells us how much iodine had been produced in the.

of the Iodine Clock Reaction Intro & Theory YouTube
The Kinetics of the Iodine Clock Reaction Pre-lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. The questions should be answered on a separate (new) page of your lab notebook. Be sure to show all work, round answers, and include units on all answers.

IODINE CLOCK REACTION Instant Color Change Experiment YouTube
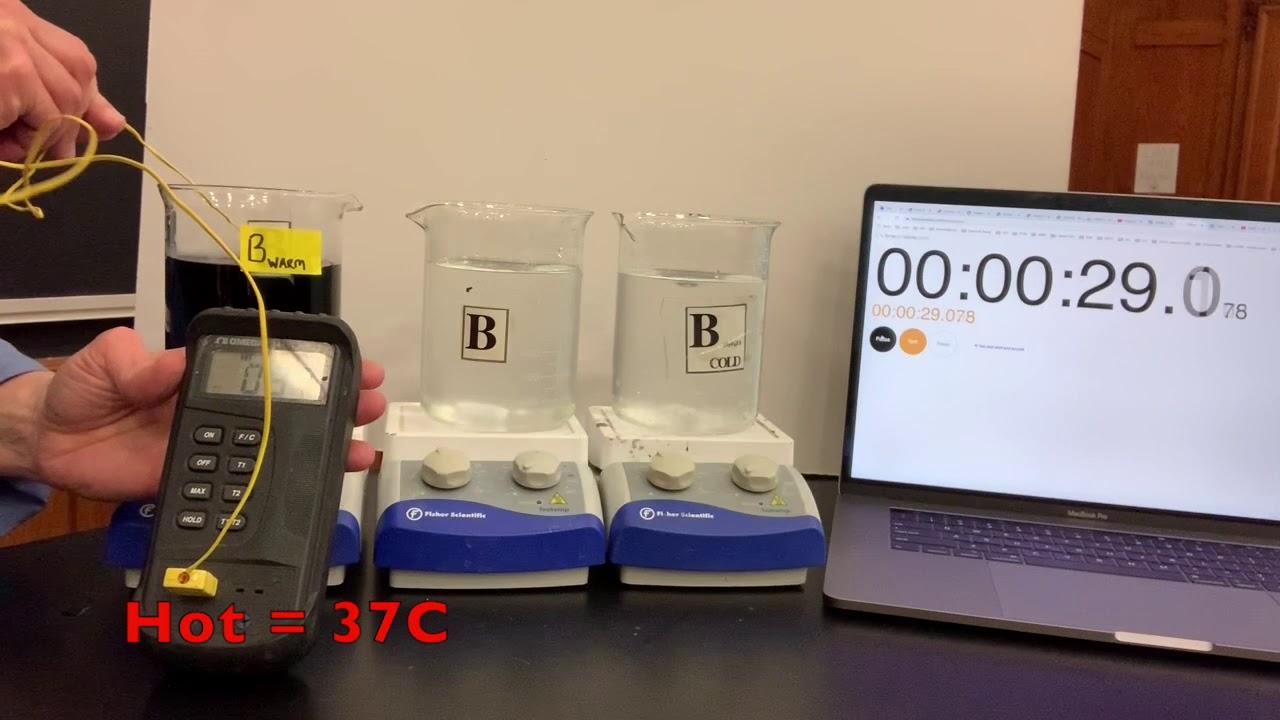
Obtain and mix the reagents in the same volumes as in step 1 for the room temperature solution. Step 2. Place the beaker in a water bath with ice. Measure the temperature on the solution. Leave the solution in the bath until the temperature is at least 10o 10 o C colder than the room temperature solution. Step 3.
iodine clock lab YouTube
A Sample Lab Report The Iodine Clock Reaction Introduction: The factors that affect the rate of a chemical reaction are important to understand due to the importance of many such reactions to our health, well-being and comfort.

Iodine Clock Reaction—Chemical Demonstration Kit Flinn Scientific
The classic iodine clock reaction demonstrates the properties of chemical kinetics through its mesmerizing change in color, and it is sure to fascinate you and perhaps your audience. With just a few household items, you can easily perform this experiment with great success. Steps Download Article 1 Gather the materials. You will essentially need:

The Iodine Clock Reaction Lab ⋆ Chemistry
Procedure: In a 400 mL beaker, add 100 mL 0.1M KIO 3, 5 mL 1% starch, and 100 mL H 2 O. In a 600 mL beaker, put in 20 mL 0.25M NaHSO 3, and 130 mL H 2 O. Mix the two solutions and after a short delay, the clear solution will instantly turn a dark blue/black ( ~ 10 seconds)

The Iodine Clock Reaction Lab ⋆ Chemistry
The iodine clock reaction exists in several variations, which each involve iodine species ( iodide ion, free iodine, or iodate ion) and redox reagents in the presence of starch. Two colourless solutions are mixed and at first there is no visible reaction.

Iodine Clock Reaction (8.1.5) AQA A Level Chemistry Revision Notes
Iodine Clock Reaction | Imagination Station Open Today: 10:00 AM - 5:00 PM Open Today: 10:00 AM - 5:00 PM Theater Open Today: 10:15 AM - 4:15 PM Directions & Parking Visit Admission Admission & Hours Directions & Parking Accessibility School Field Trips Think Tank Workshops Tinkering in IDEA Lab Science2GO! Barry Bagels KeyBank Discovery Theater

Make the Iodine Clock Reaction (Chemistry) YouTube
The iodine clock reaction is a favorite demonstration reaction in chemistry classes that usually requires toxic or hazardous chemicals. During the reaction, two clear liquids are mixed, resulting in another clear liquid. After some time, the solution suddenly turns dark blue.

The Iodine Clock Reaction Lab ⋆ Chemistry Lab
Solution Preparation Solution A 0.02 M KIO 3 Solution B 4g soluble starch, 0.2g sodium metabisulfite ( Na₂S₂O₅), 5mL 1M sulfuric acid in 1L solution Materials Electronic stop clock Ice bath in large refrigerator dish 100 mL cylinders 400 mL beakers Magnetic stirrer and stirring bars for both beakers Procedure
TemperatureDependent Iodine Clock Lecture Demonstration YouTube
Experiment 15 . Iodine Clock . E15-2 . The Task . The goal of this experiment is to identify the rate-determining step in a complex reaction mechanism. Skills At the end of the laboratory session you should be able to: • determine the concentration dependence of the rate of a reaction via the method of

How to Perform the Iodine Clock Reaction 11 Steps (with Pictures)
The purpose of this experiment was to find the Rate Law Equation, the Rate Law Constant, and the rate orders of the reactants in the reaction between two solutions; Solution A and Solution B. Solution A consisted of hydrogen peroxide, hydrogen ions, and water, and Solution B consisted of iodine ions, thiosulfate ions, and starch.

Iodine Clock experiment explained (Grade 12 school science lab) YouTube
Iodine Clock Reaction Lab Learning Objectives Practice laboratory techniques of safely altering the temperature of a solution and creating different concentrations of a solution. Observe and record the effect of changing the temperature of a system on the rate of a reaction

Classic Chemistry Colorize Colorless Liquids with "Black" Magic, AKA
Solution A: 2.1 g potassium iodate (V) is dissolved in 1 dm 3 deionised water followed by the addition of 10 cm 3 of 1 mol dm -3 sulfuric acid. Solution B: 4 g of soluble starch is made up into a paste with a little cold water and this is added to 1 dm 3 boiling deionised water. 0.9 g of sodium hydrogensulfite and 10 cm 3 of 1 mol dm -3.

How to Make an Iodine Clock Reaction at Home « Science Experiments
The Iodine Clock Reaction is a classic chemistry experiment that demonstrates many basic principles of kinetics and redox chemistry. For this, the reaction persists as a staple of general chemistry lab demonstrations. In this experiment, you prepare two simple, transparent solutions.

"Iodine clock" experiment (the BriggsRauscher reaction) MEL Chemistry
17 Kinetics of the Iodine Clock Reaction Purpose To determine the rate law and activation energy of a iodine clock reaction. Expected Learning Outcomes After completing this experiment, it is expected that students will be able to Determine the rate law and rate constant of a reaction.